Research
We are motivated by a general observation in biology: Evolution proceeds unevenly: whereas some traits and lineages appear relatively inert for millions of years, others diversify exceptionally rapidly. But why is this so? What causes evolution to achieve overdrive in some cases and straddle the slow lane in others? In this lab, we investigate the abiotic and biotic factors that guide the rate and pattern of evolution in reptiles, amphibians, and fishes. We are integrative organismal biologists: we study the phenotype as an integrated unit, and consider the organism within it environmental context. Research in my lab can be organized under three complementary themes:
I. Behavior is a motor and a brake for evolution.
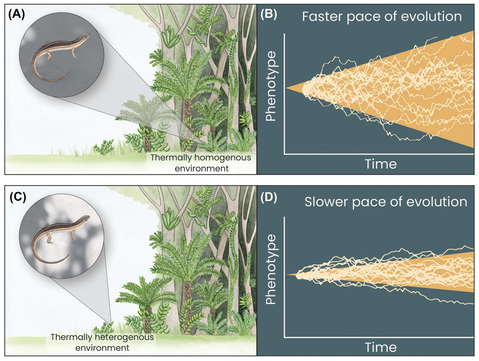 Figure: A visualization of the Bogert effect using reptile thermoregulation as an example. In panel A, a lizard behaviorally interacts with a thermally homogenous environment in closed-canopy forests (indicated by limited sun/shade patchiness in the inset). In turn, thermal homogeneity limits lizards’ ability to behaviorally thermoregulate: when translated over long time periods, such lineages should
be exposed to stronger selection on physiology, and exhibit faster physiological evolution (panel B). By contrast, more thermally heterogenous habitats, like forest edges or open habitat (panel C), provide greater opportunities for thermoregulation (indicated by greater sun/shade patchiness in the inset). When translated over long time periods, such buffering behaviors should limit exposure to selection and slow the rate of physiological evolution (i.e., the Bogert effect) (panel D). Figure from Muñoz (2022).
Figure: A visualization of the Bogert effect using reptile thermoregulation as an example. In panel A, a lizard behaviorally interacts with a thermally homogenous environment in closed-canopy forests (indicated by limited sun/shade patchiness in the inset). In turn, thermal homogeneity limits lizards’ ability to behaviorally thermoregulate: when translated over long time periods, such lineages should
be exposed to stronger selection on physiology, and exhibit faster physiological evolution (panel B). By contrast, more thermally heterogenous habitats, like forest edges or open habitat (panel C), provide greater opportunities for thermoregulation (indicated by greater sun/shade patchiness in the inset). When translated over long time periods, such buffering behaviors should limit exposure to selection and slow the rate of physiological evolution (i.e., the Bogert effect) (panel D). Figure from Muñoz (2022).
Through behavior organisms shape their evolution by dictating the selective pressures they experience. Behaviors that shield organisms from selection retard evolution (‘behavioral inertia’ or the ‘Bogert effect’) and those that expose organisms to selection accelerate evolution (‘behavioral drive’). Research in my lab centers on discovering the mechanisms that predict whether and how rapidly these phenomena unfold. Homeostatic behaviors, like thermo- and hydroregulation, shield organisms from selection and slow rates of physiological evolution. Yet, the relative magnitude of this Bogert effect is context-dependent: physiological evolution is slower in environments that reduce the costs of regulatory behavior and for traits that can be more readily buffered by behavior. In addition to the rate of evolution, the Bogert effect influences adaptive shifts in thermal physiology. The evolutionary signatures of homeostatic behaviors extend beyond physiology: behaviors that shield selection on physiology may do so by exposing organisms to selection for other traits, such as morphology. Likewise, thermoregulatory strategy influences energetic demand during activity, in turn influencing life history evolution.
Representative Papers:
Muñoz, MM (2022). The Bogert effect, a factor in evolution. Evolution. 76-S1:49-66
Salazar JC, Castañeda MR, Londoño GA, *Bodensteiner BL, Muñoz MM. (2019). Physiological evolution during adaptive radiation: A test of the island effect in Anolis lizards. Evolution. 73:1241-1252.
Muñoz MM, Losos JB. (2018). Thermoregulation simultaneously promotes and forestalls evolution in a tropical lizard. American Naturalist. 191: E15-E26
Representative Papers:
Muñoz, MM (2022). The Bogert effect, a factor in evolution. Evolution. 76-S1:49-66
Salazar JC, Castañeda MR, Londoño GA, *Bodensteiner BL, Muñoz MM. (2019). Physiological evolution during adaptive radiation: A test of the island effect in Anolis lizards. Evolution. 73:1241-1252.
Muñoz MM, Losos JB. (2018). Thermoregulation simultaneously promotes and forestalls evolution in a tropical lizard. American Naturalist. 191: E15-E26
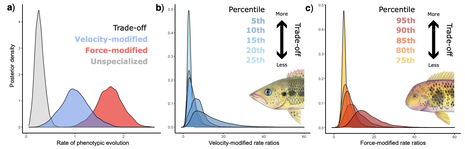
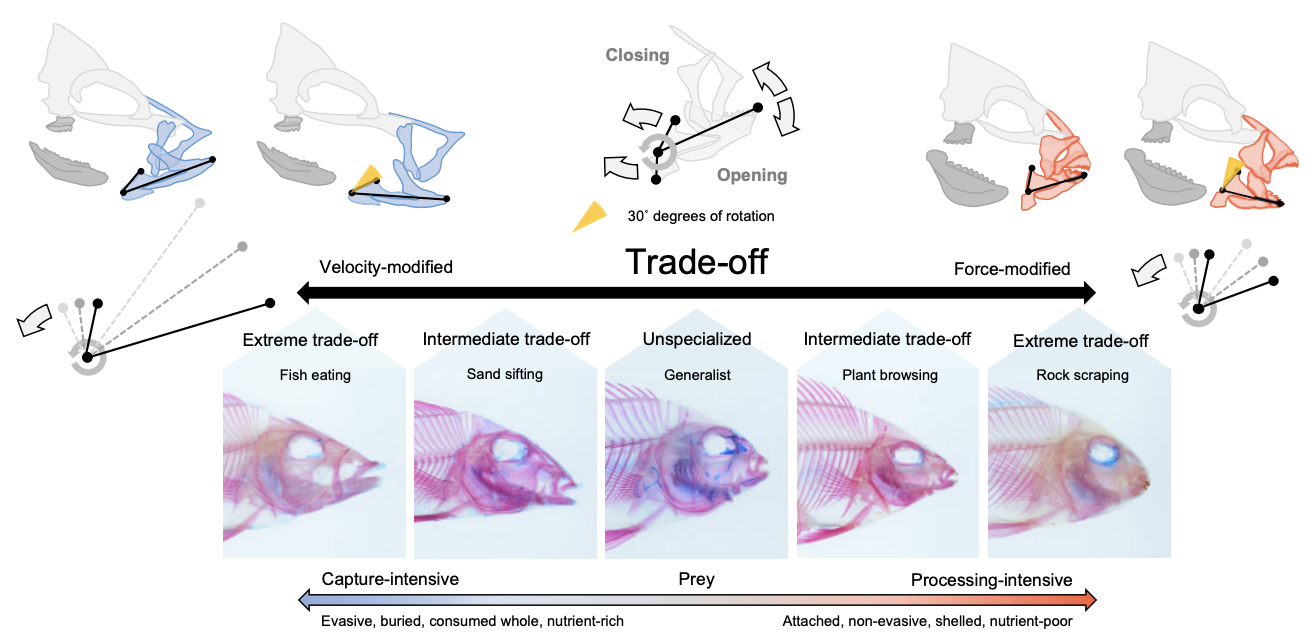
II. Mechanical relationships set the pace for phenotypic evolution.
III. Functional innovations are catalysts for evolution.
Functional innovations unlock access to novel niche space and, correspondingly, are viewed as catalysts for evolution. However, innovations do not always evolve in relative isolation; rather, multiple innovations may arise in quick succession, or confluent with other factors (e.g., geographic transitions) that interfere or synergize with the innovation. Such multiplicity can obscure the evolutionary signature of innovations, a challenge we overcome through contemporary phylogenetic approaches, and share some examples here: (1) Contrary to prevailing dogma, the invasion of Caribbean islands did not enhance the rate of trait evolution or speciation in anole lizards. Rather, the origin of adhesive toe pads, a functional innovation, was associated with a pulse of rapid speciation at the base of the anole tree, with no detectable ‘island effect’ compounding this signature. (2) Functional innovations (e.g., life cycle evolution, miniaturization, ballistic tongue feeding, among others) prompt pulses of accelerated phenotypic evolution in salamanders. When such innovations are confluent with shifts in elevation across mountains, the magnitude of the rate shift increases. In this regard, mountains and innovations interact synergistically to accelerate phenotypic evolution.
Representative Papers:
Burress ED and Muñoz MM. (2022). Ecological Opportunity from Innovation, not Islands, Drove the Anole Lizard Adaptive Radiation. Systematic Biology. 71(1):93-104.
Burress ED and Muñoz MM. (2022). Ecological Opportunity from Innovation, not Islands, Drove the Anole Lizard Adaptive Radiation. Systematic Biology. 71(1):93-104.